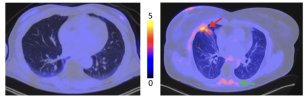
[68Ga]FAPI PET/CT reveals increased pulmonary fibroblast activation protein expression in long COVID patients after ICU discharge
B. van Leer, M. Londema, Ö. Kasalak, J. H. van Snick, M. L. Duiverman, J. C. Kuijvenhoven, M. D. de Kruif, D. E. Oprea-Lager, K. Pabst, M. E. Hellemons, H. H. Boersma, M. Prokop, M. W. Nijsten, A. W. J. M. Glaudemans, J. Pillay, R. H. J. A. Slart on behalf of the COVID-CLIMATE consortium
[Line breaks added]
Purpose
Post-acute sequelae of COVID-19 (PASC) has emerged as a major healthcare problem. A comprehensive mechanism of disease remains to be elucidated. In this study we aimed to explore pulmonary and muscle fibroblast activation protein (FAP) activity in former critical COVID-19 patients with persistent dyspnea, using [68Ga]FAPI-46 PET/CT.
Methods
In this single center prospective observational study we included former critical COVID-19 patients reporting complaints of dyspnea > 3 months after hospital discharge. A [68Ga]FAPI PET/CT scan was performed including a high-resolution CT scan, lung function test, EQ-5D questionnaire, 6 min walking test and inflammatory markers.
Age and sex-matched subjects, without pulmonary pathology, served as controls. The [68Ga]FAPI uptake was corrected for lean body mass and the target-to-background ratio (TBR) was calculated.
Results
Eighteen PASC patients and 15 controls (median age 59 and 63 years and BMI of 34.6 and 25.2 kg/m2) were included. The interval between hospital discharge and study visit was 30 months.
Increased pulmonary FAP expression was observed in PASC, (TBR 0.79 ± 0.23) compared to controls (TBR 0.40 ± 0.13, P < 0.001). Increased FAP expression was also observed in the paravertebral muscles (PASC: TBR 1.17 and controls TBR 1.00, P = 0.03).
Forced expiratory volume and forced vital capacity showed moderate negative correlation with the pulmonary TBR, while the percentage of ground glass opacities showed a moderate positive correlation.
Conclusion
[68Ga]FAPI PET/CT demonstrated elevated FAP expression in PASC. These findings provide insight into possible pathophysiological mechanisms of PASC and a potential new diagnostic modality.
Link | PDF (European Journal of Nuclear Medicine and Molecular Imaging) [Open Access]
B. van Leer, M. Londema, Ö. Kasalak, J. H. van Snick, M. L. Duiverman, J. C. Kuijvenhoven, M. D. de Kruif, D. E. Oprea-Lager, K. Pabst, M. E. Hellemons, H. H. Boersma, M. Prokop, M. W. Nijsten, A. W. J. M. Glaudemans, J. Pillay, R. H. J. A. Slart on behalf of the COVID-CLIMATE consortium
[Line breaks added]
Purpose
Post-acute sequelae of COVID-19 (PASC) has emerged as a major healthcare problem. A comprehensive mechanism of disease remains to be elucidated. In this study we aimed to explore pulmonary and muscle fibroblast activation protein (FAP) activity in former critical COVID-19 patients with persistent dyspnea, using [68Ga]FAPI-46 PET/CT.
Methods
In this single center prospective observational study we included former critical COVID-19 patients reporting complaints of dyspnea > 3 months after hospital discharge. A [68Ga]FAPI PET/CT scan was performed including a high-resolution CT scan, lung function test, EQ-5D questionnaire, 6 min walking test and inflammatory markers.
Age and sex-matched subjects, without pulmonary pathology, served as controls. The [68Ga]FAPI uptake was corrected for lean body mass and the target-to-background ratio (TBR) was calculated.
Results
Eighteen PASC patients and 15 controls (median age 59 and 63 years and BMI of 34.6 and 25.2 kg/m2) were included. The interval between hospital discharge and study visit was 30 months.
Increased pulmonary FAP expression was observed in PASC, (TBR 0.79 ± 0.23) compared to controls (TBR 0.40 ± 0.13, P < 0.001). Increased FAP expression was also observed in the paravertebral muscles (PASC: TBR 1.17 and controls TBR 1.00, P = 0.03).
Forced expiratory volume and forced vital capacity showed moderate negative correlation with the pulmonary TBR, while the percentage of ground glass opacities showed a moderate positive correlation.
Conclusion
[68Ga]FAPI PET/CT demonstrated elevated FAP expression in PASC. These findings provide insight into possible pathophysiological mechanisms of PASC and a potential new diagnostic modality.
Link | PDF (European Journal of Nuclear Medicine and Molecular Imaging) [Open Access]