John Mac
Senior Member (Voting Rights)
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) is an enigmatic condition characterized by fatigue that is unaided by rest and by exacerbation of symptoms after exertion (post-exertional malaise or “PEM”).
There is no definitive molecular marker or known underlying pathological mechanism for the condition.
Increasing evidence for aberrant energy metabolism suggests a role for mitochondrial dysfunction in ME/CFS.
Our objective was therefore to measure mitochondrial function and cellular stress sensing in actively metabolising patient blood cells.
We immortalized lymphoblasts isolated from 51 ME/CFS patients diagnosed according to the Canadian Consensus Criteria and an age- and gender-matched control group.
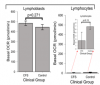
Parameters of mitochondrial function and energy stress sensing were assessed by Seahorse extracellular flux analysis, proteomics, and an array of additional biochemical assays.
As a proportion of the basal oxygen consumption rate (OCR), the rate of ATP synthesis by Complex V was significantly reduced in ME/CFS lymphoblasts, while significant elevations were observed in Complex I OCR, maximum OCR, spare respiratory capacity, nonmitochondrial OCR and “proton leak” as a proportion of the basal OCR.
This was accompanied by an elevation of mitochondrial membrane potential, chronically hyperactivated TOR Complex I stress signalling and upregulated expression of mitochondrial respiratory complexes, fatty acid transporters and enzymes of the β-oxidation and TCA cycles.
By contrast, mitochondrial mass and genome copy number, as well as glycolytic rates and steady state ATP levels were unchanged.
Our results suggest a model in which ME/CFS lymphoblasts have a Complex V defect accompanied by compensatory upregulation of their respiratory capacity that includes the mitochondrial respiratory complexes, membrane transporters and enzymes involved in fatty acid β-oxidation.
This homeostatically returns ATP synthesis and steady state levels to “normal” in resting cells, but may leave them unable to adequately respond to acute increases in energy demand as the relevant homeostatic pathways are already activated.
https://www.preprints.org/manuscript/201909.0043/v1