Now published, see post #8
-------------------------------------------
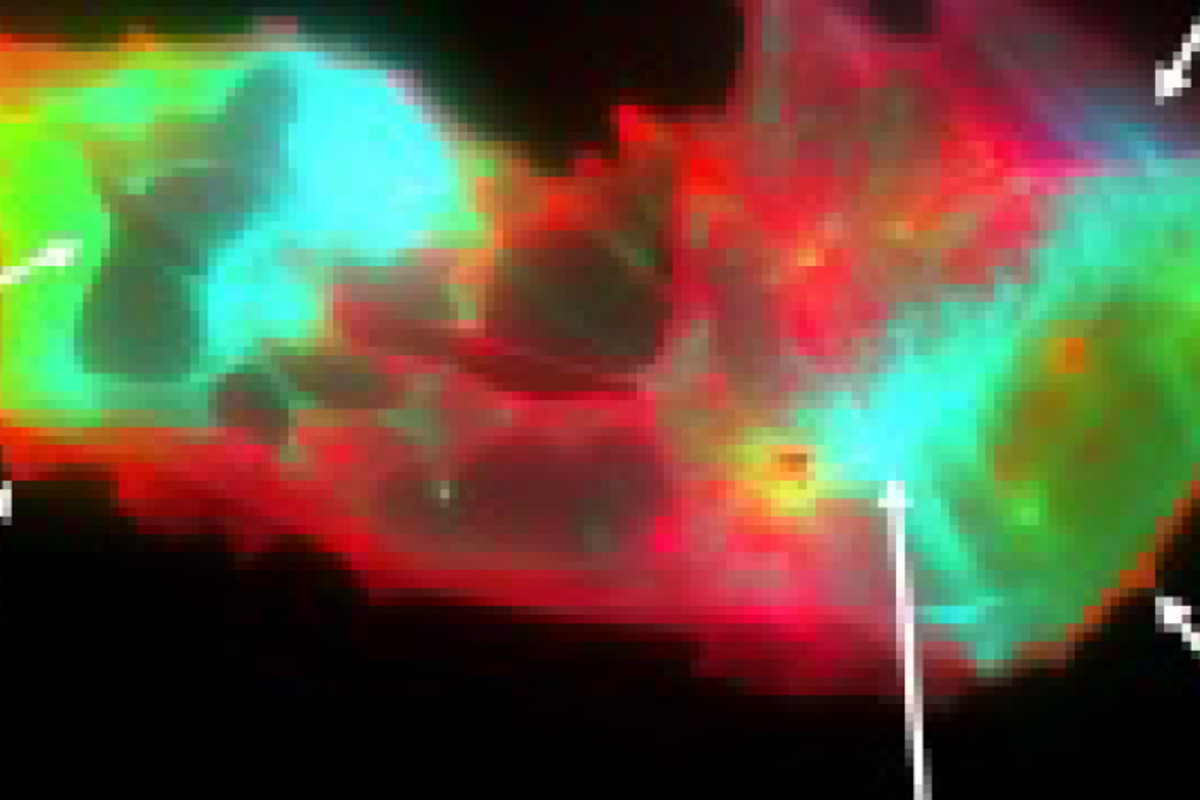
Circulating microclots are structurally associated with Neutrophil Extracellular Traps and their amounts are strongly elevated in long COVID patients
Etheresia Pretorius¹, Alain THIERRY², Cynthia Sanchez³, Tram Ha⁴, Brice Pastor⁴, Alexia Mirandola³, Ekaterina Pisareva⁵, Corinne Prevostel⁶, Gert Laubscher⁷, Tom Usher¹, Chantelle Venter¹, Simone Turner¹, Maxine Waters¹, Douglas Kell⁸
BACKGROUND: The persistence of vasculo-thrombotic complications has been put forward as a possible contributing factor in the long COVID (LC) syndrome.
OBJECTIVES: Given the recently reported separate demonstration of the association of LC with elevated levels of fibrin amyloid microclots (FAM) and with those neutrophil extracellular traps (NETs), markers that are linked to thromboinflammation, this study considers the association of FAM with NETs.
RESULTS: The results show that NETs markers are quantitatively and structurally associated with the size and number of FAM in patients with LC. These markers showed a strong diagnostic performance, both independently and when combined.
CONCLUSIONS: Our study revealed that NETs may be a component of circulating FAM, We suggest that higher NETs formation promotes the stabilization of FAM in the circulation, leading to deleterious effects which contribute causally to the LC syndrome.
Competing interests
ART, EEP and BP are author of a patent: NEW METHOD TO DIAGNOSE INFLAMMATORY DISEASES 11194720 PCT application number PCT/EP2022/072147, Date of receipt 05 August 2022. EP is an author of a patent DIAGNOSTIC METHOD FOR LONG COVID PCT application number GB2105644.5. EP is a founding director of Biocode Technologies, a Stellenbosch University start-up company. ST and AT are employees of Biocode Technologies, as well as postgraduate students of EP. All other authors: no competing interests to declare.
Link (Preprint, Research Square) [Open Access]
-------------------------------------------
Circulating microclots are structurally associated with Neutrophil Extracellular Traps and their amounts are strongly elevated in long COVID patients
Etheresia Pretorius¹, Alain THIERRY², Cynthia Sanchez³, Tram Ha⁴, Brice Pastor⁴, Alexia Mirandola³, Ekaterina Pisareva⁵, Corinne Prevostel⁶, Gert Laubscher⁷, Tom Usher¹, Chantelle Venter¹, Simone Turner¹, Maxine Waters¹, Douglas Kell⁸
- Stellenbosch University
- INSERM / IRCM
- IRCM, Montpellier Cancer Research Institute, INSERM U1194, Montpellier University
- INSERM
- IRCM, Institut de Recherche en Cancé
- Institute of Cancer Research of Montpellier
- Mediclinic Stellenbosch, Stellenbosch, 7600, South Africa
- University of Liverpool
BACKGROUND: The persistence of vasculo-thrombotic complications has been put forward as a possible contributing factor in the long COVID (LC) syndrome.
OBJECTIVES: Given the recently reported separate demonstration of the association of LC with elevated levels of fibrin amyloid microclots (FAM) and with those neutrophil extracellular traps (NETs), markers that are linked to thromboinflammation, this study considers the association of FAM with NETs.
RESULTS: The results show that NETs markers are quantitatively and structurally associated with the size and number of FAM in patients with LC. These markers showed a strong diagnostic performance, both independently and when combined.
CONCLUSIONS: Our study revealed that NETs may be a component of circulating FAM, We suggest that higher NETs formation promotes the stabilization of FAM in the circulation, leading to deleterious effects which contribute causally to the LC syndrome.
Competing interests
ART, EEP and BP are author of a patent: NEW METHOD TO DIAGNOSE INFLAMMATORY DISEASES 11194720 PCT application number PCT/EP2022/072147, Date of receipt 05 August 2022. EP is an author of a patent DIAGNOSTIC METHOD FOR LONG COVID PCT application number GB2105644.5. EP is a founding director of Biocode Technologies, a Stellenbosch University start-up company. ST and AT are employees of Biocode Technologies, as well as postgraduate students of EP. All other authors: no competing interests to declare.
Link (Preprint, Research Square) [Open Access]
Last edited: