Sly Saint
Senior Member (Voting Rights)
Increased gut permeability and bacterial translocation are associated with fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome: implications for disease-related biomarker discovery
Background: There is growing evidence of the significance of gastrointestinal complaints in the impairment of the intestinal mucosal barrier function and inflammation in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome. However, data on intestinal permeability and gut barrier dysfunction in FM and ME/CFS are still limited with conflicting results. This study aimed to assess circulating biomarkers potentially related to intestinal barrier dysfunction and bacterial translocation and their association with self-reported symptoms in these conditions.
Methods: A pilot multicentre, cross-sectional cohort study with consecutive enrolment of 22 patients with FM, 30 with ME/CFS, and 26 matched healthy controls. Plasma levels of anti-beta-lactoglobulin antibodies (IgG anti-beta-LGB), zonulin-1 (ZO-1), LPS, sCD14, and IL-1β) were assayed using ELISA. Demographic and clinical characteristics of the participants were recorded using validated self-reported outcome measures. The diagnostic accuracy of each biomarker was assessed using the ROC curve analysis.
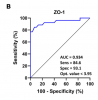
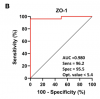
Results: FM patients had significantly higher levels of anti-β-LGB, ZO-1, LPS, and sCD14 than healthy controls (all P < 0.0001). In ME/CFS patients, levels of anti-β-LGB, ZO-1, LPS, and sCD14 were significantly higher than controls, but lower than in FM (all P < 0.01), while there was no significant difference in IL-1β level. In the FM and ME/CFS cohorts, both anti-β-LGB and ZO-1 correlated significantly with LPS and sCD14 (P < 0.001 for both). In the FM group, both anti-beta-LGB and ZO-1 were correlated significantly with physical and mental health components on the SF-36 scale (P < 0.05); whereas IL-1beta negatively correlated with the COMPASS-31 score (P < 0.05). In the ME/CFS cohort, ZO-1 was positively correlated with the COMPASS-31 score (P < 0.05). The ROC curve analysis indicated a strong ability of anti-β-LGB, ZO-1, LPS, and sCD14 to predictively distinguish between FM and ME/CFS from healthy controls (P < 0.0001).
Conclusions: Biomarkers of intestinal barrier function and inflammation were associated with autonomic dysfunction assessed by COMPASS-31 scores in FM and ME/CFS respectively. Anti-β-LGB antibodies, ZO-1, LPS, and sCD14 may be putative predictors of intestinal barrier dysfunction in these cohorts. Further studies are needed to assess whether these findings are causal and can therefore be applied in clinical practice.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1253121/abstract
Background: There is growing evidence of the significance of gastrointestinal complaints in the impairment of the intestinal mucosal barrier function and inflammation in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome. However, data on intestinal permeability and gut barrier dysfunction in FM and ME/CFS are still limited with conflicting results. This study aimed to assess circulating biomarkers potentially related to intestinal barrier dysfunction and bacterial translocation and their association with self-reported symptoms in these conditions.
Methods: A pilot multicentre, cross-sectional cohort study with consecutive enrolment of 22 patients with FM, 30 with ME/CFS, and 26 matched healthy controls. Plasma levels of anti-beta-lactoglobulin antibodies (IgG anti-beta-LGB), zonulin-1 (ZO-1), LPS, sCD14, and IL-1β) were assayed using ELISA. Demographic and clinical characteristics of the participants were recorded using validated self-reported outcome measures. The diagnostic accuracy of each biomarker was assessed using the ROC curve analysis.
Results: FM patients had significantly higher levels of anti-β-LGB, ZO-1, LPS, and sCD14 than healthy controls (all P < 0.0001). In ME/CFS patients, levels of anti-β-LGB, ZO-1, LPS, and sCD14 were significantly higher than controls, but lower than in FM (all P < 0.01), while there was no significant difference in IL-1β level. In the FM and ME/CFS cohorts, both anti-β-LGB and ZO-1 correlated significantly with LPS and sCD14 (P < 0.001 for both). In the FM group, both anti-beta-LGB and ZO-1 were correlated significantly with physical and mental health components on the SF-36 scale (P < 0.05); whereas IL-1beta negatively correlated with the COMPASS-31 score (P < 0.05). In the ME/CFS cohort, ZO-1 was positively correlated with the COMPASS-31 score (P < 0.05). The ROC curve analysis indicated a strong ability of anti-β-LGB, ZO-1, LPS, and sCD14 to predictively distinguish between FM and ME/CFS from healthy controls (P < 0.0001).
Conclusions: Biomarkers of intestinal barrier function and inflammation were associated with autonomic dysfunction assessed by COMPASS-31 scores in FM and ME/CFS respectively. Anti-β-LGB antibodies, ZO-1, LPS, and sCD14 may be putative predictors of intestinal barrier dysfunction in these cohorts. Further studies are needed to assess whether these findings are causal and can therefore be applied in clinical practice.
https://www.frontiersin.org/articles/10.3389/fimmu.2023.1253121/abstract