Evergreen
Senior Member (Voting Rights)
This is a retrospective review of medical files of 201 people with ME/CFS who were prescribed LDN, continued to take it, and provided follow-up data.
Here's what they did:
Here's what they did:
In some of the initially evaluated patients, the ME/CFS diagnosis was based on the Fukuda CFS criteria [Citation12]; however, most patients were diagnosed using the Canadian criteria [Citation13].
Treatment. The patients were directed to introduce LDN in a morning dose of 1.5 mg for one week, and then continue with a daily dose of 3.0 mg (1.5 mg twice daily). After six weeks on LDN they were allowed to increase the daily dose to 4.5 mg. Patients were informed about the most common adverse symptoms (nausea, insomnia, worsening of pain) that may appear during the initiation of LDN therapy. They were also told that the efficacy and safety of LDN had not been assessed in systematic studies, and the medication might have adverse effects that have not been previously described. If no treatment effect was observed within the first 6 months, LDN was discontinued.
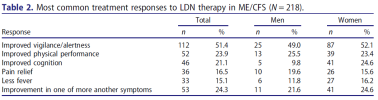
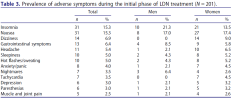
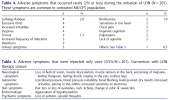
Treatment responses and adverse effects were registered on control visits or upon renewal of prescriptions. Detailed information about both treatment responses and adverse effects collected during LDN treatment was compiled from patient medical reports.
Serious adverse effects (SAEs) were not reported, although 7.3% of the patients discontinued treatment because of adverse symptoms. The most common reason for discontinuation was nausea (5 patients, 2.5%). Increased anxiety was the reason for early termination in two patients (1.0%).