Thanks
@DMissa.
To be clear, I'm impressed with lots of things about this paper so far and I very much appreciate the transparency and readability of the paper and you being here to discuss it. I'm not suggesting you have aimed to misrepresent data, I know you wouldn't do that. I chose the wrong word in 'shoehorning'.
But, when I look at the 1B chart and then read that it is evidence of a possible steroid hormone biosynthesis dysregulation in ME/CFS, it doesn't seem quite right. So, I'm trying to understand.
Edit - There was a decision to undertake and present the pathway analysis on the polar metabolites despite not identifying any polar metabolite dysregulation, so there was a choice made there.
We decided to proceed with a subsequent pathway analysis of the polar metabolite data using a feature inclusion threshold of p < 0.05 as only a brief indicative exercise to ensure that any potential processes of note weren’t overlooked as possible false negatives
Missing values
This is only for the metaboanalyst polar metabolite pathway analysis, the individual features I am showing in the paper and analysing elsewhere are all real data, no imputation.
So, Figure 1B is just the actual values, and any imputed values aren't shown? How many missing values were imputed for the three polar metabolites in the Metaboanalyst metabolite pathway analysis?
Is it possible that the inclusion of imputed values affected the p values for the pathways? For example, if, as is possible under that rule of allowing up to 50% imputed values, a metabolite had a large number of missing values, and mean values were used to replace the missing values, wouldn't that improve the p values? The extra data clustering at each of the group means would surely make the groups look more different.
That's great that the lipid PC(O:38-4) chart consists only of actual values, no imputed values. I'm really looking forward to getting to the discussion about that and I hope you can get support for replication.
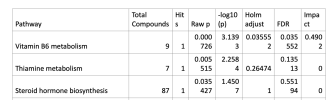
Steroid hormone biosynthesis
432 MetaboAnalyst Pathway analysis suggests possible effects on vitamin B6 metabolism, thiamine metabolism and steroid hormone biosynthesis
I'm glad the suggestion of a dysregulated steroid hormone biosynthesis didn't make it into the discussion or the abstract, but I'm still a bit concerned that that sentence in the Results (edit - it's actually a title in the paper) overstates the situation. The paper says
418 No polar metabolites satisfied the threshold for significance (FDR < 0.05) in ME/CFS LCLs using the Benjamini-Hochberg procedure for multiple comparison correction (23).
In any case, we identified no clear polar metabolite dysregulation in these LCLs
So, the possibility of a pathway being dysregulated seems to be based on only one polar metabolite that is actually not in itself significantly different, that isn't itself dysregulated. I get that if there were 4 metabolites that weren't themselves quite significant enough, but that were all in the same pathway, a valid case could be made for the pathway being dysregulated. But, when it is written that only one metabolite is driving the identification of a pathway, and that one metabolite is not significantly different between the ME/CFS cells and the controls, the lack of difference seems to be a problem.
The chart shows that the ME/CFS levels of cholesterol sulphate fit entirely within the range of the healthy controls - there are healthy control cells with both more and with less cholesterol sulphate than the ME/CFS cells. So yes, many things are technically possible, but it surely is extremely unlikely on the basis of that chart that that metabolite is the driver of a steroid hormone biosynthesis dysfunction in ME/CFS?
Figure 1B

Was there an adjustment for multiple comparisons in the MetaboAnalyst Pathway analysis?
Age
Regarding the age thing, I did look for relationships between age and total lipid and didn't see evidence of an effect. From memory I also did it for the altered features reported in the paper and didn't detect any relationships either.
It would be great if you could check the relationship between the actual values and age for LCL cholesterol sulphate levels.