Nightsong
Senior Member (Voting Rights)
Abstract:
Background/Objectives: The aetiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), a chronic and severe debilitating disease with a complex phenotype, remains elusive. Associations with infectious diseases and autoimmune and neuropsychiatric disorders have been observed, without the identification of mechanisms. Previous studies suggest that genetic predisposition plays a role, but results are difficult to replicate, with Genome-Wide Association Studies of ME/CFS being challenging due to the relative rareness and heterogeneity of the disorder.
Methods: We studied a well-defined Australian patient cohort diagnosed via the International Consensus Criteria, recruited by a specialist ME/CFS clinic. The whole-exome sequences of 77 patients were contrasted against genome variation in the 1000 Genome Project’s genome-matched population.
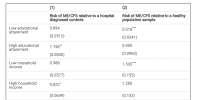
Results: Significant associations with ME/CFS were harboured in genes that belong to the Neuroblastoma Breakpoint Family encoding Olduvai (DUF1220) domains, namely NBPF1 (rs3897177, p-value = 3.15 × 10−8), NBPF10 (rs1553120233, p-value = 9.262 × 10−13), and NBPF16 (rs200632836, p-value = 1.04 × 10−6). Other significantly associated variants were detected in the ATR, RSPH10B,ADGRE5-CD97, and NTRK2 genes, among others. Replication of these results was attempted via a GWAS on raw data from a US cohort, which confirmed shared significant associations with variation identified in the PTPRD, CSMD3, RAPGEF5, DCC, ALDH18A1, GALNT16, UNC79, and NCOA3 genes.
Conclusions: These genes are involved in cortical neurogenesis, brain evolution, and neuroblastoma, and have been implicated by several studies in schizophrenia and autism. The sharing of these associations by the two cohorts supports their validity and grants the necessity of future studies to evaluate the implications for ME/CFS aetiology.
https://www.mdpi.com/2075-4418/15/12/1542
Background/Objectives: The aetiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), a chronic and severe debilitating disease with a complex phenotype, remains elusive. Associations with infectious diseases and autoimmune and neuropsychiatric disorders have been observed, without the identification of mechanisms. Previous studies suggest that genetic predisposition plays a role, but results are difficult to replicate, with Genome-Wide Association Studies of ME/CFS being challenging due to the relative rareness and heterogeneity of the disorder.
Methods: We studied a well-defined Australian patient cohort diagnosed via the International Consensus Criteria, recruited by a specialist ME/CFS clinic. The whole-exome sequences of 77 patients were contrasted against genome variation in the 1000 Genome Project’s genome-matched population.
Results: Significant associations with ME/CFS were harboured in genes that belong to the Neuroblastoma Breakpoint Family encoding Olduvai (DUF1220) domains, namely NBPF1 (rs3897177, p-value = 3.15 × 10−8), NBPF10 (rs1553120233, p-value = 9.262 × 10−13), and NBPF16 (rs200632836, p-value = 1.04 × 10−6). Other significantly associated variants were detected in the ATR, RSPH10B,ADGRE5-CD97, and NTRK2 genes, among others. Replication of these results was attempted via a GWAS on raw data from a US cohort, which confirmed shared significant associations with variation identified in the PTPRD, CSMD3, RAPGEF5, DCC, ALDH18A1, GALNT16, UNC79, and NCOA3 genes.
Conclusions: These genes are involved in cortical neurogenesis, brain evolution, and neuroblastoma, and have been implicated by several studies in schizophrenia and autism. The sharing of these associations by the two cohorts supports their validity and grants the necessity of future studies to evaluate the implications for ME/CFS aetiology.
https://www.mdpi.com/2075-4418/15/12/1542