Neurovascular injury with complement activation and inflammation in COVID-19
Myoung-Hwa Lee, Daniel P. Perl, Joseph Steiner, Nicholas Pasternack, Wenxue Li, Dragan Maric, Farinaz Safavi, Iren Horkayne-Szakaly, Robert Jones, Michelle N. Stram, Joel T. Moncur, Marco Hefti, Rebecca D. Folkerth, Avindra Nath
The underlying mechanisms by which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to acute and long-term neurological manifestations remains obscure. We aimed to characterize the neuropathological changes in patients with coronavirus disease 2019 and determine the underlying pathophysiological mechanisms.
In this autopsy study of the brain, we characterized the vascular pathology, the neuroinflammatory changes and cellular and humoral immune responses by immunohistochemistry.
All patients died during the first wave of the pandemic from March to July 2020. All patients were adults who died after a short duration of the infection, some had died suddenly with minimal respiratory involvement. Infection with SARS-CoV-2 was confirmed on ante-mortem or post-mortem testing. Descriptive analysis of the pathological changes and quantitative analyses of the infiltrates and vascular changes were performed.
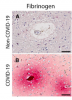
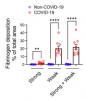
All patients had multifocal vascular damage as determined by leakage of serum proteins into the brain parenchyma. This was accompanied by widespread endothelial cell activation. Platelet aggregates and microthrombi were found adherent to the endothelial cells along vascular lumina. Immune complexes with activation of the classical complement pathway were found on the endothelial cells and platelets. Perivascular infiltrates consisted of predominantly macrophages and some CD8+ T cells. Only rare CD4+ T cells and CD20+ B cells were present. Astrogliosis was also prominent in the perivascular regions. Microglial nodules were predominant in the hindbrain, which were associated with focal neuronal loss and neuronophagia.
Antibody-mediated cytotoxicity directed against the endothelial cells is the most likely initiating event that leads to vascular leakage, platelet aggregation, neuroinflammation and neuronal injury. Therapeutic modalities directed against immune complexes should be considered.
Link to DOI | PDF
Myoung-Hwa Lee, Daniel P. Perl, Joseph Steiner, Nicholas Pasternack, Wenxue Li, Dragan Maric, Farinaz Safavi, Iren Horkayne-Szakaly, Robert Jones, Michelle N. Stram, Joel T. Moncur, Marco Hefti, Rebecca D. Folkerth, Avindra Nath
The underlying mechanisms by which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to acute and long-term neurological manifestations remains obscure. We aimed to characterize the neuropathological changes in patients with coronavirus disease 2019 and determine the underlying pathophysiological mechanisms.
In this autopsy study of the brain, we characterized the vascular pathology, the neuroinflammatory changes and cellular and humoral immune responses by immunohistochemistry.
All patients died during the first wave of the pandemic from March to July 2020. All patients were adults who died after a short duration of the infection, some had died suddenly with minimal respiratory involvement. Infection with SARS-CoV-2 was confirmed on ante-mortem or post-mortem testing. Descriptive analysis of the pathological changes and quantitative analyses of the infiltrates and vascular changes were performed.
All patients had multifocal vascular damage as determined by leakage of serum proteins into the brain parenchyma. This was accompanied by widespread endothelial cell activation. Platelet aggregates and microthrombi were found adherent to the endothelial cells along vascular lumina. Immune complexes with activation of the classical complement pathway were found on the endothelial cells and platelets. Perivascular infiltrates consisted of predominantly macrophages and some CD8+ T cells. Only rare CD4+ T cells and CD20+ B cells were present. Astrogliosis was also prominent in the perivascular regions. Microglial nodules were predominant in the hindbrain, which were associated with focal neuronal loss and neuronophagia.
Antibody-mediated cytotoxicity directed against the endothelial cells is the most likely initiating event that leads to vascular leakage, platelet aggregation, neuroinflammation and neuronal injury. Therapeutic modalities directed against immune complexes should be considered.
Link to DOI | PDF
Last edited: