Now published - see post
Preprint
Post-COVID syndrome is associated with capillary alterations, macrophage infiltration and distinct transcriptomic signatures in skeletal muscles
Tom Aschman, Emanuel Wyler, Oliver Baum, Andreas Hentschel, Franziska Legler, Corinna Preusse, Lil Meyer-Arndt, Ivana Buettnerova, Alexandra Foerster, Derya Cengiz, Luiz Gustavo Teixeira Alves, Julia Schneider, Claudia Kedor, Rebekka Rust, Judith Bellmann-Strobl, Aminaa Sanchin, Peter Vajkoczy, Hans-Hilmar Goebel, Markus Landthaler, Victor Corman, Andreas Roos, Frank L. Heppner, Helena Radbruch, Friedemann Paul, Carmen Scheibenbogen, Werner Stenzel, Nora F. Dengler
The SARS-CoV-2 pandemic not only resulted in millions of acute infections worldwide, but also caused innumerable cases of post-infectious syndromes, colloquially referred to as long COVID. Due to the heterogeneous nature of symptoms and scarcity of available tissue samples, little is known about the underlying mechanisms.
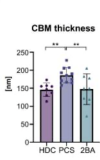
We present an in-depth analysis of skeletal muscle biopsies obtained from eleven patients suffering from enduring fatigue and post-exertional malaise after an infection with SARS-CoV-2. Compared to two independent historical control cohorts, patients with post-COVID exertion intolerance had fewer capillaries, thicker capillary basement membranes and increased numbers of CD169+ macrophages. SARS-CoV-2 RNA could not be detected in the muscle tissues, but transcriptomic analysis revealed distinct gene signatures compared to the two control cohorts, indicating immune dysregulations and altered metabolic pathways.
We hypothesize that the initial viral infection may have caused immune-mediated structural changes of the microvasculature, potentially explaining the exercise-dependent fatigue and muscle pain.
Link
Preprint
Post-COVID syndrome is associated with capillary alterations, macrophage infiltration and distinct transcriptomic signatures in skeletal muscles
Tom Aschman, Emanuel Wyler, Oliver Baum, Andreas Hentschel, Franziska Legler, Corinna Preusse, Lil Meyer-Arndt, Ivana Buettnerova, Alexandra Foerster, Derya Cengiz, Luiz Gustavo Teixeira Alves, Julia Schneider, Claudia Kedor, Rebekka Rust, Judith Bellmann-Strobl, Aminaa Sanchin, Peter Vajkoczy, Hans-Hilmar Goebel, Markus Landthaler, Victor Corman, Andreas Roos, Frank L. Heppner, Helena Radbruch, Friedemann Paul, Carmen Scheibenbogen, Werner Stenzel, Nora F. Dengler
The SARS-CoV-2 pandemic not only resulted in millions of acute infections worldwide, but also caused innumerable cases of post-infectious syndromes, colloquially referred to as long COVID. Due to the heterogeneous nature of symptoms and scarcity of available tissue samples, little is known about the underlying mechanisms.
We present an in-depth analysis of skeletal muscle biopsies obtained from eleven patients suffering from enduring fatigue and post-exertional malaise after an infection with SARS-CoV-2. Compared to two independent historical control cohorts, patients with post-COVID exertion intolerance had fewer capillaries, thicker capillary basement membranes and increased numbers of CD169+ macrophages. SARS-CoV-2 RNA could not be detected in the muscle tissues, but transcriptomic analysis revealed distinct gene signatures compared to the two control cohorts, indicating immune dysregulations and altered metabolic pathways.
We hypothesize that the initial viral infection may have caused immune-mediated structural changes of the microvasculature, potentially explaining the exercise-dependent fatigue and muscle pain.
Link
Last edited by a moderator: