Andy
Senior Member (Voting rights)
Preprint.
Paper now published, see this post
Abstract
A blood-based diagnostic test for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and multiple sclerosis (MS) would be of great value in both conditions, facilitating more accurate and earlier diagnosis, helping with current treatment delivery, and supporting the development of new therapeutics.
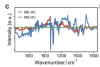
Here we use Raman micro-spectroscopy to examine differences between the spectral profiles of blood cells of ME/CFS, MS and healthy controls. We were able to discriminate the three groups using ensemble classification models with high levels of accuracy (91%) with the additional ability to distinguish mild, moderate, and severe ME/CFS patients from each other (84%). To our knowledge, this is the first research using Raman micro-spectroscopy to discriminate specific subgroups of ME/CFS patients on the basis of their symptom severity. Specific Raman peaks linked with the different disease types with the potential in further investigations to provide insights into biological changes associated with the different conditions.
https://www.medrxiv.org/content/10.1101/2023.03.18.23286575v1
Paper now published, see this post
Abstract
A blood-based diagnostic test for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and multiple sclerosis (MS) would be of great value in both conditions, facilitating more accurate and earlier diagnosis, helping with current treatment delivery, and supporting the development of new therapeutics.
Here we use Raman micro-spectroscopy to examine differences between the spectral profiles of blood cells of ME/CFS, MS and healthy controls. We were able to discriminate the three groups using ensemble classification models with high levels of accuracy (91%) with the additional ability to distinguish mild, moderate, and severe ME/CFS patients from each other (84%). To our knowledge, this is the first research using Raman micro-spectroscopy to discriminate specific subgroups of ME/CFS patients on the basis of their symptom severity. Specific Raman peaks linked with the different disease types with the potential in further investigations to provide insights into biological changes associated with the different conditions.
https://www.medrxiv.org/content/10.1101/2023.03.18.23286575v1
Last edited by a moderator: