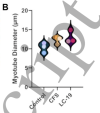
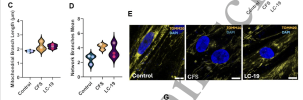
I'm just jumping ahead to Methods to check that the cohorts are not wildly different on confounders.
Age, sex (all female) and BMI matched.
ME/CFS - ICC, specialist confirmed diagnosis
LC - confirmed Covid-19 infection, also ICC with PEM, at least 3 months after the acute infection
Sedentary donors
That's all sounding fine.
Age, sex (all female) and BMI matched.
ME/CFS - ICC, specialist confirmed diagnosis
LC - confirmed Covid-19 infection, also ICC with PEM, at least 3 months after the acute infection
Sedentary donors
That's all sounding fine.
I think that means that the various serum samples were allocated randomly to the fabricated muscle. They don't say that the investigators were blinded to which cohort a sample came from, so it doesn't sound as though the assessment was really blinded. They didn't know which cohort was meant to perform worse, but I don't think it would have been hard to make a guess about that.For construct treatment experiments, serum biosamples were randomly assigned to the control or treatment groups. Investigators performing data analysis were blinded to the study hypotheses.